Background: Patients (pts) with R/R MCL and TP53 mutation/deletion, or high Ki-67 proliferation index (PI), have historically had limited treatment options with dismal outcomes. Brexu-cel is a CAR T-cell therapy approved in the US for adults with R/R MCL. Real-world data has been consistent with that of clinical trials (Kambhampati et al. 2023). In a 3-year follow-up of ZUMA-2 (Wang et al. 2022), outcomes were comparable across various high-risk subgroups (TP53 mutation, Ki-67 PI ≥ 30% or ≥ 50%). Here, we describe real-world outcomes of brexu-cel in R/R MCL by high-risk features, including deletion of TP53 or 17p, Ki-67 PI, and by ZUMA-2 eligibility.
Methods : A total of 500 pts receiving brexu-cel for R/R MCL from 84 US centers between 07/2020−12/2022 were prospectively enrolled in the CIBMTR observational database for a post-authorization safety study. In this analysis, 446 pts were included, excluding pts with prior non-transplant cellular therapy, missing data for analysis, or no follow-up. Effectiveness outcomes were overall response rate (ORR), complete response (CR) rate, duration of response (DOR), progression-free survival (PFS), overall survival (OS) and relapse/progressive disease (REL/PD). Safety outcomes included cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), prolonged cytopenias, infections requiring treatment, subsequent neoplasms, and non-relapse mortality (NRM). Analyses conducted included univariate as well as multivariate logistic regression and Cox proportional hazard models fit to identify covariates with an impact on outcomes.
Results : Of the 446 pts included and who had available data, 20% (44/220) had deletion of TP53/17p at diagnosis; Ki-67 PI ≥ 50% at diagnosis was seen in 42% (107/252). Pts with deletion of TP53/17p were less likely to undergo prior autologous hematopoietic cell transplant (11% vs 30%). Pts with Ki-67 PI ≥ 50% were more likely to receive Bruton's tyrosine kinase inhibitor (BTKi) (92% vs 81%) and bridging therapy (52% vs 37%), but less likely to receive bendamustine (47% vs 62%). Pts with either high-risk feature tended to have a shorter time from diagnosis to brexu-cel infusion. Overall, 67% (297/446) of pts would not have met ZUMA-2 eligibility criteria, mainly due to pulmonary impairment (33%), cardiac impairment (21%), prior malignancy (21%), low platelet count (20%), and no prior BTKi (18%).
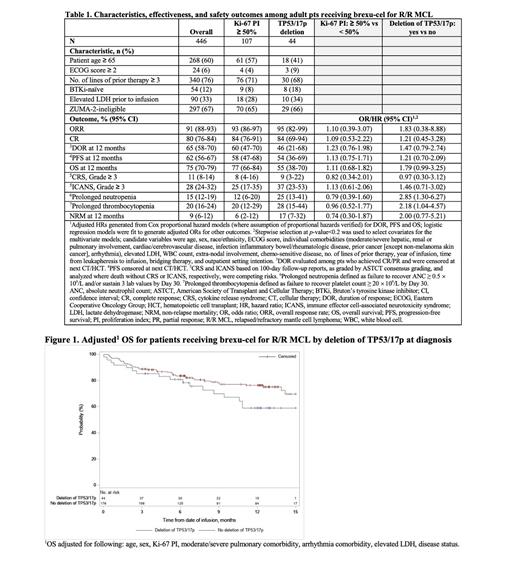
With a median follow-up of 12.2 mo, DOR, PFS and OS were evaluated at 12 mo. CR was 84% (ORR, 95%; DOR, 46%; PFS, 54%; OS, 55%) for pts with deletion of TP53/17p vs 80% (ORR, 90%; DOR, 65%; PFS, 61%; OS, 77%) in those without. CR for pts with Ki-67 PI ≥ 50% vs < 50% was 84% (ORR, 93%; DOR, 60%; PFS, 58%; OS, 77%) vs 83% (ORR, 92%; DOR, 69%; PFS, 63%; OS, 73%); for pts eligible vs ineligible to ZUMA-2, CR was 84% (ORR, 92%; DOR, 73%; PFS, 70%; OS, 82%) vs 79% (ORR, 90%; DOR, 60%; PFS, 57%; OS, 71%). Safety endpoints were largely consistent among all subgroups. Prolonged neutropenia and thrombocytopenia occurred more frequently in pts with vs without deletion of TP53/17p (25% vs 12% and 28% vs 17%, respectively). Grade ≥ 3 CRS occurred more frequently in ZUMA-2-ineligible vs eligible pts (13% vs 7%).
After multivariable adjustment, all effectiveness and most safety outcomes were consistent regardless of deletion of TP53/17p. Deletion of TP53/17p approached an association with decreased OS (HR 1.79 [95% CI 0.99−3.25]), and was associated with more frequent prolonged neutropenia (OR 2.85 [1.30−6.27]) and prolonged thrombocytopenia (OR 2.18 [1.04−4.57]) although these did not result in significant difference in infections requiring treatment (HR 1.22 [0.79−1.90]) nor NRM (HR 2.00 [0.77−5.21]). The remaining outcomes were consistent regardless of deletion of TP53/17p. Comparable outcomes were observed between pts with Ki-67 PI ≥ 50% and < 50%.
Conclusions: These real-world findings with 12 mo of follow-up suggest that outcomes of brexu-cel treatment are largely consistent, including a high CR rate, regardless of ZUMA-2 eligibility or the high-risk feature subgroups analyzed. Although pts without deletion of TP53/17p appeared to have longer OS than pts with deletion of TP53/17p, the data further support brexu-cel as the standard of care across pts with R/R MCL, including those with high-risk features. An updated dataset is planned to be analyzed and results can be presented at the conference.
*SK and NA are equal contributors.
Disclosures
Ahmed:Kite, a Gilead company: Research Funding; Bristol Myers Squibb: Consultancy. Hamadani:Sanofi Genzyme: Speakers Bureau; ADC therapeutics: Consultancy, Honoraria, Research Funding, Speakers Bureau; Myeloid Therapeutics: Honoraria; Astra Zeneca: Speakers Bureau; Astellas: Research Funding; SeaGen: Consultancy; MorphoSys: Consultancy; Novartis: Consultancy; Legend Biotech: Consultancy; Genmab: Consultancy; Kadmon: Consultancy; Incyte: Consultancy; Gamida Cell: Consultancy; BeiGene: Speakers Bureau; Kite, a Gilead Company: Consultancy, Speakers Bureau; AstraZeneca: Speakers Bureau; Caribou: Consultancy; Bristol Myers Squibb: Consultancy; Genmab: Consultancy; CRISPR: Consultancy; Omeros: Consultancy; Abbvie: Consultancy; BeiGene: Speakers Bureau; Spectrum Pharmaceuticals: Research Funding; Takeda: Research Funding; Genentech: Honoraria. Hemmer:Kite, a Gilead Company: Current Employment, Research Funding; Pfizer: Consultancy, Honoraria; Karyopharm: Consultancy; Takeda: Consultancy; Tubulis: Consultancy; Regeneron: Consultancy; Genmab: Consultancy; Caribou: Consultancy; Adicet Bio: Consultancy; Abbvie: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; ADC therapeutics: Research Funding; Merck: Research Funding; Seattle Genetics: Research Funding. Grover:Tessa Therapeutics: Research Funding; Caribou Biosciences: Honoraria; Seagen: Honoraria; Genentech: Honoraria; Kite: Honoraria; ADC Therapeutics: Consultancy, Honoraria; Novartis: Honoraria; Sangamo: Current holder of stock options in a privately-held company; Seattle Genetics: Consultancy. Shadman:Genentech: Consultancy, Research Funding; Janssen: Consultancy; ADC therapeutics: Consultancy; Fate Therapeutics: Consultancy; Genmab: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; MorphoSys/Incyte: Consultancy, Research Funding; Vincerx: Research Funding; Mustang Bio: Consultancy, Research Funding; Eli Lilly: Consultancy; Pharmacyclics: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy; BeiGene: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; MEI Pharma: Consultancy; Regeneron: Consultancy; TG Therapeutics: Research Funding. Beitinjaneh:Angiocrine: Research Funding; Tessa: Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Autolus: Research Funding; Atara: Research Funding. Budde:Roche: Consultancy; AstraZeneca: Consultancy, Research Funding; ADC Therapeutics: Consultancy; Amgen: Research Funding; Merck: Research Funding; Novartis, Gilead, F. Hoffmann-La Roche Ltd, BeiGene, Genentech, Inc.: Consultancy; MustangBio: Research Funding. Gerson:Loxo Oncology: Research Funding; AbbVie: Consultancy; Genentech: Consultancy. Jacobson:Synthekine: Consultancy; Morphosys: Consultancy; AstraZeneca: Consultancy; Ipsen: Consultancy; Daiichi-Sankyo: Consultancy; Abbvie: Consultancy; Bristol Myers Squibb/Celgene: Consultancy; Novartis: Consultancy; Caribou Bio: Consultancy; Abintus Bio: Consultancy; ImmPACT Bio: Consultancy; Instil Bio: Consultancy; Kite, a Gilead company: Consultancy, Research Funding; ADC Therapeutics: Consultancy; Pfizer: Research Funding; Miltenyi Biotec: Consultancy. Locke:Cellular Medicine Group: Consultancy; ASH: Other: Travel Support; Bluebird Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional; Emerging Therapy Solutions: Consultancy, Other; Caribou: Consultancy; Calibr: Consultancy; Pfizer: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Legend Biotech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Aptitude Health: Other: Travel Support; Society for Immunotherapy of Cancer: Other; Clinical Care Options Oncology: Other; CERo Therapeutics: Other: (Institutional); Sana: Consultancy, Membership on an entity's Board of Directors or advisory committees; Wugen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Umoja: Consultancy, Membership on an entity's Board of Directors or advisory committees; BioPharma Communications CARE Education: Other: Institutional; EcoR1: Consultancy; Iovance: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Leukemia and Lymphoma Society: Other; National Cancer Institute: Other; Daiichi Sankyo: Consultancy; Cowen: Consultancy; Imedex: Other; Allogene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional ; GammaDelta Therapeutics: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional , Research Funding; Kite, a Gilead Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional , Research Funding; Bristol Myers Squibb/ Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional , Research Funding; Gerson Lehrman Group (GLG): Consultancy; A2 Biotherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support. Wang:Medscape: Honoraria; IDEOlogy Health: Honoraria; i3Health: Honoraria; AbbVie: Consultancy, Honoraria; Genmab: Honoraria, Research Funding; Milken Institute: Consultancy; Miltenyi Biomedicine: Consultancy; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel, Research Funding; Merck: Consultancy, Honoraria; Meeting Minds Experts: Honoraria; CAHON: Honoraria; Eastern Virginia Medical School: Honoraria; Dava Oncology: Honoraria, Other: Travel; Amphista Therapeutics Limited: Consultancy; ADC Therapeutics America: Consultancy; AstraZeneca: Consultancy, Honoraria, Other: Travel, Research Funding; BioInvent: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; VelosBio: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria; Be Biopharma: Consultancy; Acerta Pharma: Consultancy, Honoraria, Research Funding; Deciphera: Consultancy; Leukemia & Lymphoma Society: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Physicians Education Resources (PER): Honoraria, Other: Travel; OncLive: Honoraria; Oncology Specialty Group: Honoraria; Nurix: Honoraria; NIH: Honoraria; Moffit Cancer Center: Honoraria; MJH Life Sciences: Honoraria; MD Education: Honoraria; Janssen: Consultancy, Honoraria, Research Funding; InnoCare: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Oncternal: Consultancy, Research Funding; DTRM Biopharma (Cayman) Limited: Consultancy; Parexel: Consultancy; Pepromene Bio: Consultancy; Pharmacyclics: Consultancy, Honoraria, Research Funding; Bantam Pharmaceutical: Honoraria; Eli Lilly and Company: Consultancy, Research Funding; Practice Point Communications (PPC): Honoraria; Scripps: Honoraria; Studio ER Congressi: Honoraria; WebMD: Honoraria; Celgene: Other: Travel, Research Funding; Juno Therapeutics: Research Funding; Genentech: Consultancy, Research Funding; Loxo Oncology: Consultancy, Research Funding; Molecular Templates: Research Funding; Vincerx: Research Funding; Anticancer Association: Honoraria; BGICS: Honoraria; Clinical Care Options: Honoraria; Epizyme: Consultancy, Honoraria; Hebei Cancer Prevention Federation: Honoraria; Imedex: Honoraria; TS Oncology: Honoraria; Mumbai Hematology Group: Honoraria; OMI: Honoraria; Pharmacyclics: Honoraria; Physicians Education Resources: Honoraria; Practice Point Communications: Honoraria; CSTone: Consultancy. Yan:Gilead Sciences: Current holder of stock options in a privately-held company; Kite, A Gilead Company: Current Employment. Nunes:Gilead Sciences Europe Ltd: Current Employment, Current holder of stock options in a privately-held company, Honoraria; Amgen: Current holder of stock options in a privately-held company. Dalton:Bristol Myers Squibb: Current holder of stock options in a privately-held company, Other; Gilead Sciences: Current holder of stock options in a privately-held company, Other; Kite, a Gilead Company: Current Employment. Wu:Viracta: Current holder of stock options in a privately-held company, Other; Curis: Current holder of stock options in a privately-held company, Other; Avid: Current holder of stock options in a privately-held company, Other; Amgen: Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company, Divested equity in a private or publicly-traded company in the past 24 months, Other; AbbVie: Current holder of stock options in a privately-held company, Other; Abbott: Current holder of stock options in a privately-held company, Other; Gilead Sciences: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company, Divested equity in a private or publicly-traded company in the past 24 months, Other. Abdeldaim:Kite, a Gilead Company: Current Employment, Other: Travel Support; Gilead Sciences: Current holder of stock options in a privately-held company. Hu:Kite, a Gilead Company: Current Employment; Gilead Sciences: Current holder of stock options in a privately-held company. Pasquini:Novartis: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Kite, a Gilead Company: Honoraria, Research Funding; Janssen: Research Funding; Kite Brazil: Honoraria. Herrera:ADC Therapeutics: Consultancy, Research Funding; Tubulis GmbH: Consultancy; Kite, a Gilead Company: Research Funding; Genmab: Consultancy; BMS: Consultancy, Other: Travel/Accommodations/Expenses, Research Funding; AbbVie: Consultancy; Takeda: Consultancy; AstraZeneca/MedImmune: Consultancy; Adicet Bio: Consultancy; Genentech/Roche: Consultancy, Research Funding; Merck: Consultancy, Research Funding; Karyopharm Therapeutics: Consultancy; Pfizer: Consultancy; Regeneron: Consultancy; Seattle Genetics: Consultancy, Research Funding; Caribou Biosciences: Consultancy; Allogene Therapeutics: Consultancy; Gilead Sciences: Research Funding; AstraZeneca: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal